If you have questions about any of these classes, or about classes available in the region, please contact our Preparedness, Exercise, and Training Coordinator, Ryanne Holland, at [email protected] or 804-723-0511 ext. 5. Thank you!
Author Archive: cvhc
Strategies for PPE During COVID – 19
Healthcare systems across Central Virginia and the United States are experiencing personal protective equipment (PPE) supply chain challenges. Due to decreases in PPE exports from impacted countries and increases in demand as a result of the COVID-19 outbreak, manufacturers of select types of PPE- including N95 respirators and facemasks- are reporting increased volume of orders and challenges in meeting order demands . While plans are underway to surge PPE manufacturing globally, current impacts include delayed shipping and reduced fulfillment of PPE orders across all healthcare provider types and geographic regions.
The Central Virginia Healthcare Coalition (CVHC) is continuing to monitor and share information regarding the supply of PPE with healthcare organizations in the region. However, CVHC has no mechanism to affect the vendor prioritization, fulfillment, or distribution of PPE orders on behalf of its stakeholders. The Commonwealth of Virginia has requested release of PPE from federal stockpiles to augment supplies in primary and outpatient care settings. However, no disposition on that request is available at this time. Healthcare organizations should continue to contact their primary PPE vendor for updates on order status. For awareness, a consolidated listing of additional PPE vendors and their contact information has been attached to this message. As this situation remains fluid and dynamic, please continue to share information regarding PPE availability, needs, and shortages with CVHC by posting on the VHASS COVID-19 Event Log using your login to VHASS.
PPE Burn Rate Calculator
| This is a spreadsheet-based model that provides information for healthcare facilities to plan and optimize the use of PPE for response to coronavirus disease 2019 (COVID-19). We recommend downloading and saving the PPE burn rate calculator spreadsheet to your computer before opening the spreadsheet. Taking this step will open the spreadsheet in Excel rather than your web browser (CDC). |
| Personal Protective Equipment (PPE) Burn Rate Calculator |
Contingency and crisis strategies are based upon these assumptions:
- The facility understands its PPE inventory and supply chain.
- The facility understands their PPE utilization rate.
- The facility has been in communication with their Regional Healthcare Coalition. (If you have not requested PPE from CVHC and you are located in our region please use this form)
- Facilities have already implemented other engineering and administrative control measures including:
- Reducing the number of patients going to hospitals or outpatient settings
- Excluding non-essential HCP from entering patient care areas
- Reducing face-to-face HCP encounters with patients
- Excluding visitors to patients with confirmed or suspected COVID-19
- Cohorting patients and HCP
- Maximizing use of telemedicine
- The facilities have provided HCP with required education and training, including having them demonstrate competency with donning and doffing, with any PPE ensemble that is used to perform job responsibilities, such as patient care.
Eye Protection
Use eye protection according to product labeling and local, state, and federal requirements.
Selectively cancel elective and non-urgent procedures and appointments for which eye protection is typically used by HCP.
Shift eye protection supplies from disposable to re-usable devices (i.e., goggles and reusable face shields).
Implement extended use of eye protection.
Cancel all elective and non-urgent procedures and appointments for which eye protection is typically used by HCP.
Use eye protection devices beyond the manufacturer-designated shelf life during patient care activities.
Prioritize eye protection for selected activities
Consider using safety glasses (e.g., trauma glasses) that have extensions to cover the side of the eyes.
Exclude HCP at higher risk for severe illness from COVID-19 from contact with known or suspected COVID-19 patients.
Designate convalescent HCP for provision of care to known or suspected COVID-19 patients.
The practice of wearing the same eye protection for repeated close contact encounters with several different patients, without removing eye protection between patient encounters. Extended use of eye protection can be applied to disposable and reusable devices.
Reprocessing is the act of cleaning an item and reusing it again. Always adhere to recommended manufacturer instructions for cleaning and disinfection.
A disposable faceshield should be dedicated to one HCP and reprocessed whenever it is visibly soiled or removed (e.g., when leaving the isolation area) prior to putting it back on.
If it becomes visibly soiled or difficult to see through
If it is damaged (e.g., face shield can no longer fasten securely to the provider), if visibility is obscured and reprocessing does not restore visibility.
- While wearing gloves, carefully wipe the inside, followed by the outside of the face shield or goggles using a clean cloth saturated with neutral detergent solution or cleaner wipe.
- Carefully wipe the outside of the face shield or goggles using a wipe or clean cloth saturated with EPA-registered hospital disinfectant solution.
- Wipe the outside of face shield or goggles with clean water or alcohol to remove residue.
- Fully dry (air dry or use clean absorbent towels).
- Remove gloves and perform hand hygiene.
Isolation Gowns
Use isolation gown alternatives that offer equivalent or higher protection.
Selectively cancel elective and non-urgent procedures and appointments for which a gown is typically used by HCP.
Shift gown use towards cloth isolation gowns.
Consider the use of coveralls.
Use of expired gowns beyond the manufacturer-designated shelf life for training.
Use gowns or coveralls conforming to international standards.
Cancel all elective and non-urgent procedures and appointments for which a gown is typically used by HCP.
Extend use of isolation gowns
Re-use cloth isolation gowns
Prioritize gowns
Reprocessing is the act of cleaning an item and reusing it again. Always adhere to recommended manufacturer instructions for cleaning and disinfection.
Consideration can be made to extend the use of isolation gowns (disposable or cloth) such that the same gown is worn by the same HCP when interacting with more than one patient known to be infected with the same infectious disease when these patients housed in the same location (i.e., COVID-19 patients residing in an isolation cohort). This can be considered only if there are no additional co-infectious diagnoses transmitted by contact (such as Clostridioides difficile) among patients. If the gown becomes visibly soiled, it must be removed and discarded as per usual practices.
Cloth isolation gowns could potentially be untied and retied and could be considered for re-use without laundering in between.
However, for care of patients with suspected or confirmed COVID-19, HCP risk from re-use of cloth isolation gowns without laundering among:
- single HCP caring for multiple patients using one gown is unclear
- among multiple HCP sharing one gown is unclear.
The goal of this strategy is to minimize exposures to HCP and not necessarily prevent transmission between patients. Any gown that becomes visibly soiled during patient care should be disposed of and cleaned.
Reusable (i.e., washable) gowns are typically made of polyester or polyester-cotton fabrics. Gowns made of these fabrics can be safely laundered according to routine procedures and reused. Care should be taken to ensure that HCP do not touch outer surfaces of the gown during care.
- Laundry operations and personnel may need to be augmented to facilitate additional washing loads and cycles
- Systems are established to routinely inspect, maintain (e.g., mend a small hole in a gown, replace missing fastening ties), and replace reusable gowns when needed (e.g., when they are thin or ripped)
Consider using gown alternatives that have not been evaluated as effective.
In situation of severely limited or no available isolation gowns, the following pieces of clothing can be considered as a last resort for care of COVID-19 patients as single use. However, none of these options can be considered PPE, since their capability to protect HCP is unknown. Preferable features include long sleeves and closures (snaps, buttons) that can be fastened and secured.
- Disposable laboratory coats
- Reusable (washable) patient gowns
- Reusable (washable) laboratory coats
- Disposable aprons
- Combinations of clothing: Combinations of pieces of clothing can be considered for activities that may involve body fluids and when there are no gowns available:
- Long sleeve aprons in combination with long sleeve patient gowns or laboratory coats
- Open back gowns with long sleeve patient gowns or laboratory coats
- Sleeve covers in combination with aprons and long sleeve patient gowns or laboratory coats
Reusable patient gowns and lab coats can be safely laundered according to routine procedures.
Facemasks
Use facemasks according to product labeling and local, state, and federal requirements.
- FDA-cleared surgical masks are designed to protect against splashes and sprays and are prioritized for use when such exposures are anticipated, including surgical procedures.
- Facemasks that are not regulated by FDA, such as some procedure masks, which are typically used for isolation purposes, may not provide protection against splashes and sprays.
Selectively cancel elective and non-urgent procedures and appointments for which a facemask is typically used by HCP.
Remove facemasks for visitors in public areas
Implement extended use of facemasks
Restrict facemasks to use by HCP, rather than patients for source control.
Cancel all elective and non-urgent procedures and appointments for which a facemask is typically used by HCP.
Use facemasks beyond the manufacturer-designated shelf life during patient care activities.
Implement limited re-use of facemasks.
Prioritize facemasks for selected activities.
The practice of wearing the same facemask for repeated close contact encounters with several different patients, without removing the facemask between patient encounters.
- The facemask should be removed and discarded if soiled, damaged, or hard to breathe through.
- HCP must take care not to touch their facemask. If they touch or adjust their facemask they must immediately perform hand hygiene.
- HCP should leave the patient care area if they need to remove the facemask.
Limited re-use of facemasks is the practice of using the same facemask by one HCP for multiple encounters with different patients but removing it after each encounter.
- The facemask should be removed and discarded if soiled, damaged, or hard to breathe through.
Facemasks that fasten to the provider via ties may not be able to be undone without tearing and should be considered only for extended use, rather than re-use.
Facemasks with elastic ear hooks may be more suitable for re-use.
Facemasks should be carefully folded so that the outer surface is held inward and against itself to reduce contact with the outer surface during storage. The folded mask can be stored between uses in a clean sealable paper bag or breathable container.
Exclude HCP at higher risk for severe illness from COVID-19 from contact with known or suspected COVID-19 patients
Designate convalescent HCP for provision of care to known or suspected COVID-19 patients
Use a face shield that covers the entire front (that extends to the chin or below) and sides of the face with no facemask
Consider use of expedient patient isolation rooms for risk reduction.
Consider use of ventilated headboards
HCP use of homemade masks
N95 Respirators
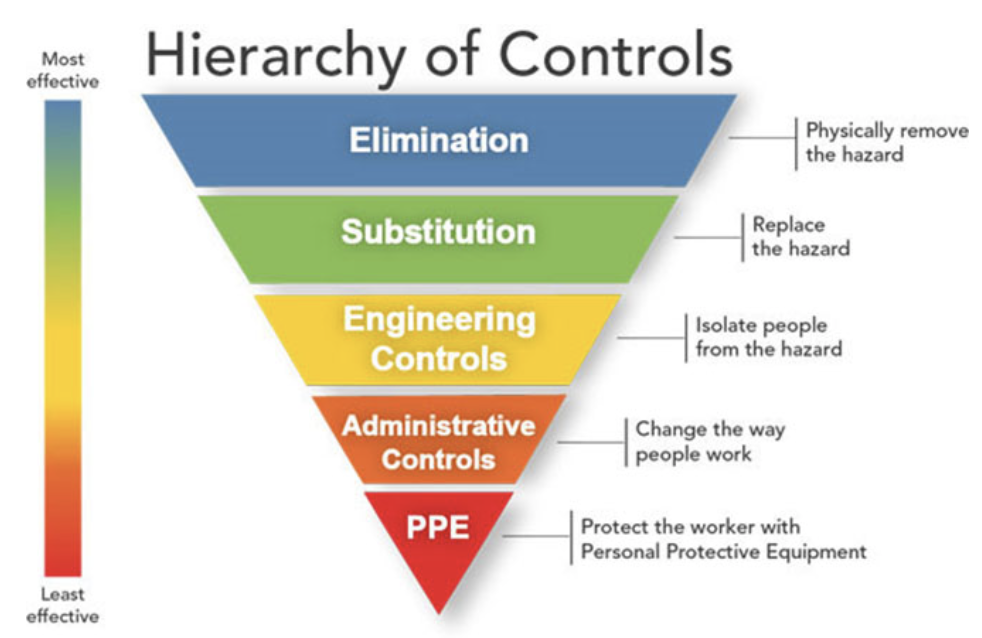
While engineering and administrative controls should be considered first when selecting controls, the use of personal protective equipment (PPE) should also be part of a suite of strategies used to protect personnel.
The use of alternative to N95 respirators:
Use alternatives to N95 respirators where feasible. These include other classes of filtering facepiece respirators, elastomeric half-mask and full facepiece air purifying respirators, powered air purifying respirators (PAPRs) where feasible. All of these alternatives will provide equivalent or higher protection than N95 respirators when properly worn. NIOSH maintains a searchable, online version of the certified equipment list identifying all NIOSH-approved respirators.
NIOSH approves other filtering facepiece respirators that are at least as protective as the N95. These include N99, N100, P95, P99, P100, R95, R99, and R100.
Elastomeric respirators are half-facepiece, tight-fitting respirators that are made of synthetic or rubber material permitting them to be repeatedly disinfected, cleaned, and reused. They are equipped with exchangeable filter cartridges. Similar to N95 respirators, elastomeric respirators require annual fit testing. Elastomeric respirators should not be used in surgical settings due to concerns that air coming out of the exhalation valve may contaminate the sterile field.
PAPRs are reusable respirators that are typically loose-fitting hoods or helmets. These respirators are battery-powered with blower that pulls air through attached filters or cartridges. The filter is typically a high-efficiency particulate air (HEPA) filter. Loose-fitting PAPRs do not require fit-testing and can be worn by people with facial hair. However, PAPRs should not be used in surgical settings due to concerns that the blower exhaust and exhaled air may contaminate the sterile field.
Use of N95 respirators beyond the manufacturer-designated shelf life for training and fit testing
Extended use of N95 respirators
Limited re-use of N95 respirators for tuberculosis
Use of respirators beyond the manufacturer-designated shelf life for healthcare delivery
Use of respirators approved under standards used in other countries that are similar to NIOSH-approved N95 respirators
Limited re-use of N95 respirators for COVID-19 patients
Use of additional respirators beyond the manufacturer-designated shelf life for healthcare delivery
Prioritize the use of N95 respirators and facemasks by activity type
Extended use refers to the practice of wearing the same N95 respirator for repeated close contact encounters with several different patients, without removing the respirator between patient encounters. Extended use is well suited to situations wherein multiple patients with the same infectious disease diagnosis, whose care requires use of a respirator, are cohorted (e.g., housed on the same hospital unit).
There is no way of determining the maximum possible number of safe reuses for an N95 respirator as a generic number to be applied in all cases. Safe N95 reuse is affected by a number of variables that impact respirator function and contamination over time. However, manufacturers of N95 respirators may have specific guidance regarding reuse of their product.
- Use a cleanable face shield (preferred) or a surgical mask over an N95 respirator and/or other steps (e.g., masking patients, use of engineering controls), when feasible to reduce surface contamination of the respirator.
Following use during aerosol generating procedures.
If it is contaminated with blood, respiratory or nasal secretions, or other bodily fluids from patients.
Following close contact with any patient co-infected with an infectious disease requiring contact precautions.
Secondary exposures can occur from respirator reuse if respirators are shared among users and at least one of the users is infectious (symptomatic or asymptomatic). Thus, N95 respirators must only be used by a single wearer.
- Hang used respirators in a designated storage area or keep them in a clean, breathable container such as a paper bag between uses. To minimize potential cross-contamination, store respirators so that they do not touch each other and the person using the respirator is clearly identified. Storage containers should be disposed of or cleaned regularly.
- Clean hands with soap and water or an alcohol-based hand sanitizer before and after touching or adjusting the respirator (if necessary for comfort or to maintain fit).
- Avoid touching the inside of the respirator. If inadvertent contact is made with the inside of the respirator, perform hand hygiene as described above.
- Use a pair of clean (non-sterile) gloves when donning a used N95 respirator and performing a user seal check. Discard gloves after the N95 respirator is donned and any adjustments are made to ensure the respirator is sitting comfortably on your face with a good seal.
Exclude HCP at higher risk for severe illness from COVID-19 from contact with known or suspected COVID-19 patients
Designate convalescent HCP for provision of care to known or suspected COVID-19 patients
Use a face shield that covers the entire front (that extends to the chin or below) and sides of the face with no facemask
Consider use of expedient patient isolation rooms for risk reduction.
Consider use of ventilated headboards
HCP use of non-NIOSH approved masks or homemade masks
- Visually inspect the N95 to determine if its integrity has been compromised.
- Check that components such as the straps, nose bridge, and nose foam material did not degrade, which can affect the quality of the fit, and seal and therefore the effectiveness of the respirator.
- If the integrity of any part of the respirator is compromised, or if a successful user seal check cannot be performed, discard the respirator and try another respirator.
- Users should perform a user seal check immediately after they don each respirator and should not use a respirator on which they cannot perform a successful user seal check.
Reference
https://www.cdc.gov/coronavirus/2019-ncov/hcp/index.html
Emerging Disease Coronavirus
Volume 2. Issue 2 – January 28, 2020
The CDC is monitoring an outbreak of a novel coronavirus (respiratory illness) named 2019-nCoV. It was first detected in Wuhan City, Hubei Province, China and believed to have originated from a live animal market. Unfortunately, it seems to have evolved from animal-to-person spread to person-to-person. This situation is still emerging and rapidly evolving and as such links will be attached below to monitor events as they occur.
Please click on this link to see information regarding the number of people under investigation.
Global Map
As of 11:00 a.m. ET January 31, 2020
Symptoms
- Fever
- Cough
- Shortness of breath
These symptoms can range from mild to severe and can appear as little as two days or as long as fourteen days after exposure.
Criteria for Persons Under Investigation (PUI)

FAQ
Yes, the first case was noted on January 21, 2020. Please click on this link to see the current count of infection.
Health care providers should contact their local/state health department immediately to notify them of patients with fever and lower respiratory illness who traveled to Wuhan, China within 14 days of symptom onset.
Criteria for Patients Under Investigation
To increase the likelihood of detecting 2019-nCoV infection, CDC recommends collecting and testing multiple clinical specimens from different sites, including all three specimen types—lower respiratory, upper respiratory, and serum specimens. Additional specimen types (e.g., stool, urine) may be collected and stored. Specimens should be collected as soon as possible once a PUI is identified regardless of time of symptom onset.
CDC’s EOC will assist local/state health departments to collect, store, and ship specimens appropriately to CDC, including during afterhours or on weekends/holidays. At this time, diagnostic testing for 2019-nCoV can be conducted only at CDC.
Although the transmission dynamics have yet to be determined, CDC currently recommends a cautious approach. Such patients should be asked to wear a surgical mask as soon as they are identified and be evaluated in a private room with the door closed, ideally an airborne infection isolation room if available. Healthcare personnel entering the room should use standard precautions, contact precautions, airborne precautions, and use eye protection (e.g., goggles or a face shield).
VDH Surveillance Data

Checklists & Forms
Resources
Novel Coronavirus 2019, Wuhan, China. (2020, January 24). Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/index.html
Virginia Department of Health. (n.d.). Retrieved from http://www.vdh.virginia.gov/surveillance-and-investigation/novel-coronavirus/
Influenza (Flu) Outbreak
Volume 2. Issue 1 – January 22, 2020
Influenza is a contagious respiratory illness caused by influenza viruses. It can range from mild to severe with serious outcomes resulting in possible hospital stays or death. Pediatrics, geriatrics, and people with compromised immune systems or certain health conditions (*diabetics, HIV/AIDS, pregnant women, asthmatics, cancer) are at high risk for serious flu complications. There are two main types of influenza: Type A and B. They are routinely spread from person to person by coughing, sneezing, or talking.
*This list is not all inclusive.
Some common signs and symptoms of the Flu can include:
- Fever
- Cough
- Sore throat
- Runny or stuffy nose
- Muscle or body aches
- Headaches
- Fatigue
Diagnosis can be confirmed by “rapid influenza diagnostic tests (RIDTs). They detect the part of the virus that stimulates an immune response. Other tests include the “rapid molecular assays” and several more accurate and sensitive tests available that are performed in specialized laboratories. The test involves a healthcare provider swiping the inside of the nose or the back of the throat with a swab.
The CDC has a weekly flu activity and surveillance page that includes surveillance data nationwide and by state. Virginia has been marked as high
Please use this link to access it: https://www.cdc.gov/flu/weekly/fluactivitysurv.htm.
The VDH also has a 2019-20 flu surveillance page specific to Virginia and can be found here: http://www.vdh.virginia.gov/epidemiology/influenza-flu-in-virginia/influenza-surveillance/
CVHC Specific
CHVC has checklists available for our different stakeholders to assist with creating a response plan for a pandemic flu response. Please feel free to download and use the plans as a guide for your particular agency.
Business Pandemic Influenza Planning Checklist
EMS Pandemic Influenza Planning Checklist
Home Healthcare Pandemic Influenza Planning Checklist
Hospital Pandemic Influenza Planning Checklist
Law Enforcement Pandemic Influenza Planning Checklist
Long Term Care Pandemic Influenza Planning Checklist
State and Local Pandemic Influenza Planning Checklist
Sources:
Influenza (Flu). (2019, December 27). Retrieved from https://www.cdc.gov/flu/index.htm.
Influenza (Flu) in Virginia. (n.d.). Retrieved December 30, 2019, from http://www.vdh.virginia.gov/epidemiology/influenza-flu-in-virginia/.
Hurricane Preparedness – Before, During, and After
Volume 1. Issue 6. – August 30, 2019
Hurricanes are large storm systems formed over warm oceans and track toward land. These systems can cause powerful winds, heavy rainfall, storm surges, flooding, increased rip currents, and the formation of tornadoes (often multiple). Secondary effects of these storms will result in power outages, downed trees and erosions or landslides.
Hurricane season runs from June 1 to November 30 for the East Coast (Atlantic) of the United States and May 15 to November 30 for the West Coast (Pacific). Hurricanes can span across >100 miles inland and are most active in September.
Prepare NOW before a hurricane, Survive during and, Stay Safe after a Hurricane!
Before a Hurricane: PREPARE
- Know your area’s risk of hurricanes.
- Become familiar with your evacuation zone, the evacuation route, and shelter locations.
- VDEM’s KNOW YOUR ZONE (click for link)
- Sign up for your community’s warning system.
- Become familiar with your evacuation zone, the evacuation route, and shelter locations.
- Stay alert to updates and warning
- Gather supplies, medicines, food for all persons and pets, to last 3 days
- Need help knowing what to put in your preparedness kit? : EmergencySuppliesKit
- Locate a safe shelter – interior room without windows on the lowest level not prone to flooding
- Practice going to the shelter / safe space for high winds
- Make a communications plan with family members
- Plan what time you will all check in and via what method (ex: text or call)
- Keep phone numbers written down in your preparedness bag incase of cellular power/use loss
- Prepare for prolonged power outages
- Fuel up your vehicles and generators
- Charge cellular phones and flashlights or medical devices
- Turn your refrigerator or freezer to the coldest setting and open only when necessary.
- Keep a thermometer in the refrigerator
- Keep important documents in a safe place or create password-protected digital copies.
- Prepare your home.
- Declutter drains and gutters.
- Install valves in plumbing to prevent backups.
- Review insurance policies.
- Bring loose yard debris, decorations, and tools inside. These items can become projectiles in high winds. Do not bring in gasoline or propane tanks.
- If you are in an immediate impact zone: Cover your home windows with permanent shutters or wood boards
During a Hurricane: SURVIVE
- Evacuate or Shelter in Place
- Protect yourself from high winds and flying debris by taking refuge in an interior room of your house/building without windows.
- Evacuate if you are advised to do so.
- If trapped in a building by flooding, go to the highest level of the building. Do not climb into a closed attic. You may become trapped by rising flood water.
- Be aware of emergency alerts and alarms
- Never use a generator indoors or near windows
- “Turn Around, Don’t Drown” – never drive/walk/swim through flood waters of an unknown level
- Stay off bridges over fast-moving water
After a Hurricane: STAY SAFE
- Listen to authorities for information and special instructions.
- Be careful during clean-up.
- Wear protective clothing and work with someone else.
- Do not touch electrical equipment if it is wet or if you are standing in water. If it is safe to do so, turn off electricity at the main breaker or fuse box to prevent electric shock.
- Avoid wading in flood water, which can contain dangerous debris. Underground or downed power lines can also electrically charge the water.
- Save phone calls for emergencies. Phone systems are often down or busy after a disaster. Use text messages or social media to communicate with family and friends.
- Stay alert for Storm Surge!
- Storm Surge is the leading cause of hurricane-related deaths in the USA (ready.gov).
- A storm surge occurs when water from the ocean is pushed toward the shore and lands by the forceful winds of the hurricane.
- Storm Surge occurs rapidly causing extreme flooding even many miles inland causing destruction of property and life.
- Storm surge can cause the undermining of highways/bridges/roads and foundations
- Assess the effects of persons and property
- Persons – seek medical care and relocation if necessary
- Property – assess and document for damage
- One inch of water in your home can cause approximately $25,000 of damage.
- Flood damage is not typically covered in basic homeowners or renter’s insurance
- Document any property damage with photographs. Contact your insurance company for assistance.
Sources:
Department of Homeland Security. Ready.gov: Hurricanes. Retrieved from https://www.ready.gov/hurricanes
Skywarn, Owlie. National Weather Service. Emergency Supplies Kit. Retrieved from https://www.weather.gov/owlie/emergencysupplieskit
Virginia Department of Emergency Management. Know Your Zone. Retrieved from https://www.vaemergency.gov/hurricane-evacuation-zone-lookup/
